Each line in the spectra represents the energy
4.6 (527) · $ 8.00 · In stock
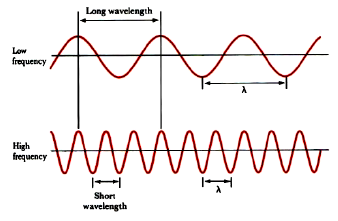
Lab 6 - Quantum States for the Visible Hydrogen Atomic Emission
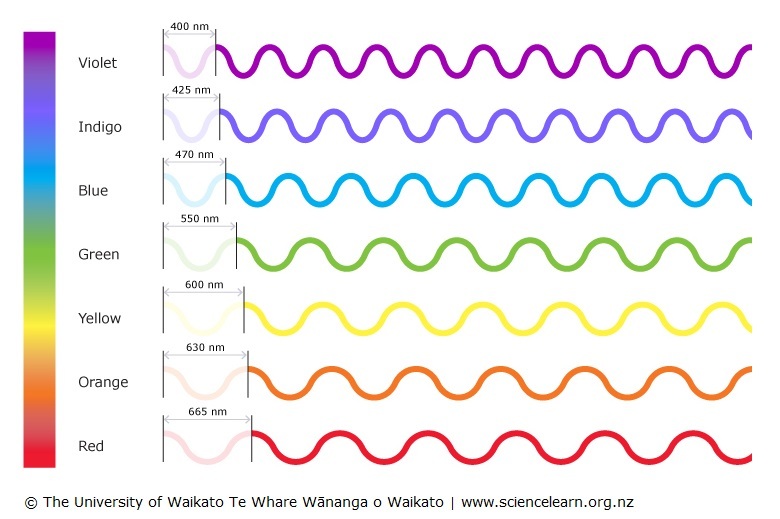
Colours of light — Science Learning Hub
Absorption spectroscopy - Wikipedia
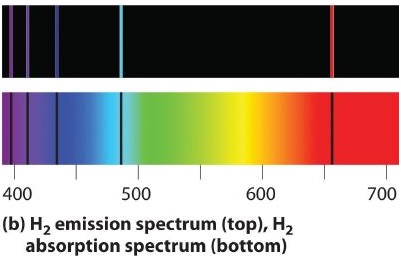
6.3: Line Spectra and the Bohr Model - Chemistry LibreTexts
Regents Chemistry Exam Explanations January 2016
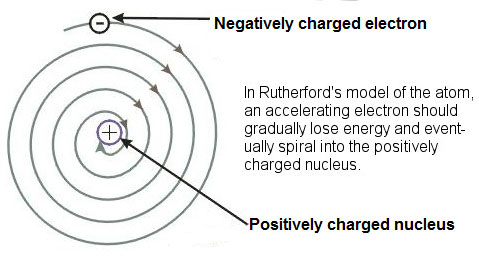
The Bohr Model

What do the lines in an emission spectrum represent? - Quora

The Periodic Table & Quantum Theory - ppt download

Castle learning of all chapters Flashcards

Spectra lines - Definition, Classification, Types, broadening

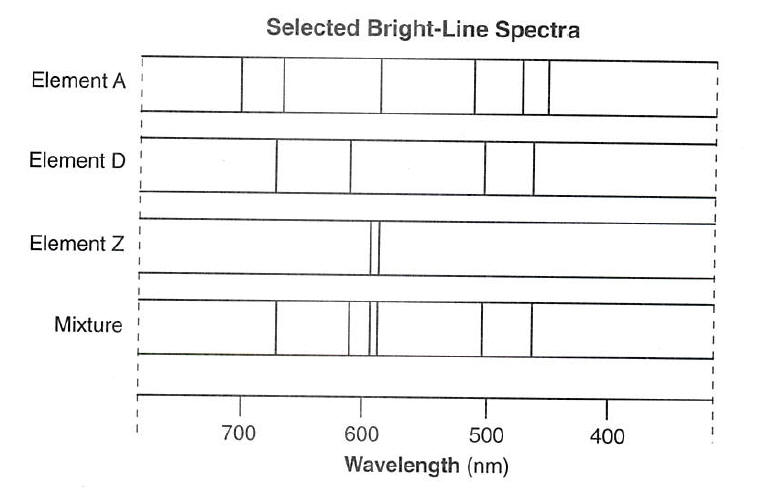
The bright-line spectra produced by four elements are represented in the diagram below. Given the bright-line spectrum of a mixture formed from two of these
You may also like






Related products






© 2018-2024, rac.tj, Inc. or its affiliates
